Are probiotics really useless? An analysis of two studies.
Two new studies released
On September 6th two studies were published in the journal Cell about the use of probiotics. The media inundated the internet with headlines like “Probiotics are useless and can actually hurt you!”
If you are one of the millions of people around the world who use probiotics to improve your health - you may be wondering if they really are useless?
Let’s look at the data in these studies to see what they really have to say about probiotics.
Why is the microbiome important?
Microbes have always co-existed with humans. In fact, the mitochondria inside our cells are actually organisms that learned to exist symbiotically with us. However, the study of the microbiome is very new. We are just beginning to understand our relationship with the trillions of bacteria that colonize our guts.
Here’s what we do know:
There are trillions of microorganisms inside every single human on this planet. There are close to 1 microorganism in your body for every human cell (1). This ratio varies person to person and changes throughout the day.
Microorganisms are found throughout the body of healthy humans. Unique species are found in each area (2).
Thousands of unique species colonize your gut microbiome (3). We’re just beginning to understand the behaviors of the most common species and how the impact our health (4).
Humans have ingested microbes through naturally fermented foods for thousands of years. Fermentation was a way to prolong the shelf life of food. It wasn’t until the early 1900’s that the health benefits of fermentation were recognized (5).
“The correct combination and concentration of gastrointestinal microflora is determined by nature and numerous interdependent variables. Changing one factor such as concentration and trying to “optimize” nature’s delicately balanced gastrointestinal environment may very well be altering a condition that nature never intended to alter. The short- and long-term effects of this change may be difficult to evaluate given the multifactorial nature of the gastrointestinal environment.”
- Amy C. Brown, Ph.D., R.D. and Ana Valiere, M.S., Probiotics and Medical Nutrition Therapy, 2006
When did people start using probiotics?
People have been eating fermented foods for thousands of years. But the first known instance of someone eating a fermented food for the purpose of improving their microbiome was Eli Metchnikoff in the early 1900’s. Metchnikoff actually received the Nobel Prize for his work in demonstrating that eating beneficial bacteria can crowd out bad bacteria in the gut (5).
Since then scientists have been studying and learning about the microbiome and how we can influence it with probiotics.
In 2007 the Human Microbiome Project began collecting and testing samples from people across the world (6). The goal is to catalog the types of microbes that coexist with humans. This is the first step in understanding how our health is affected by the trillions of microbes in and on our bodies.
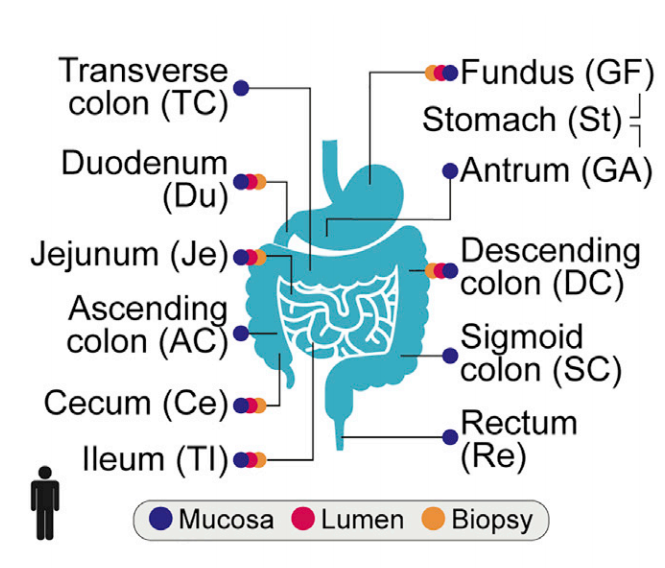
11 locations tested throughout the GI tract in order to compare gut microbe samples to stool microbe samples.
What was found in the two recent studies?
The first study published in the journal Cell is titled Personalized Gut Mucosal Colonization Resistance to Impiric Probiotics is Associated with Unique Host and Microbiome Features (7).
The first study asked the question “Is a stool sample an accurate picture of the gut microbiome?”
They enlisted 25 healthy individuals in the study. They took a stool sample from each person and performed an invasive endoscopy and colonoscopy to retrieve microbe samples from 11 different places along the digestive tract.
The 20 most common bacteria strains found in the gut. The columns correspond with the sample locations noted in the image above.
They looked at the species found in each part. For simplicity, this image shows the most common species, not all of the species found.
They took two types of samples. The Mucosa sample is what they scraped off the side of the digestive tract. The Lumen sample is what was suctioned from the general area.
Now we can compare the microbes pulled directly from the gut with microbes found in the stool. What we see is the lower GI tract, from the Ileum to the rectum, is closer to the stool sample than the upper GI. However the stool sample is not a mirror image. For example, we see Flexispira, streptococcus, and biffidobacterium in the stool, but very little actually colonized in the gut. Similarly from the stool it might look like Lachnospiraceae and bacteroides appear in approximately equal amounts, which is not at all the case in the gut samples.
This is important because stool sampling is the most popular method of analyzing the gut. Stool sampling is still helpful in a clinical setting and practitioners trained in stool tests already know that the stool is not a perfect mirror image of what’s actually happening in the gut.
As more at-home stool tests become available, it’s important to understand this information and work with a practitioner who can help you interpret the results.
Later in the study, the researchers asked the question, “do probiotics effectively colonize the gut?”
The researchers took 15 people, 5 received a placebo and 10 received probiotics. They used an 11 strain probiotic with 25 billion active bacteria, administered twice a day.
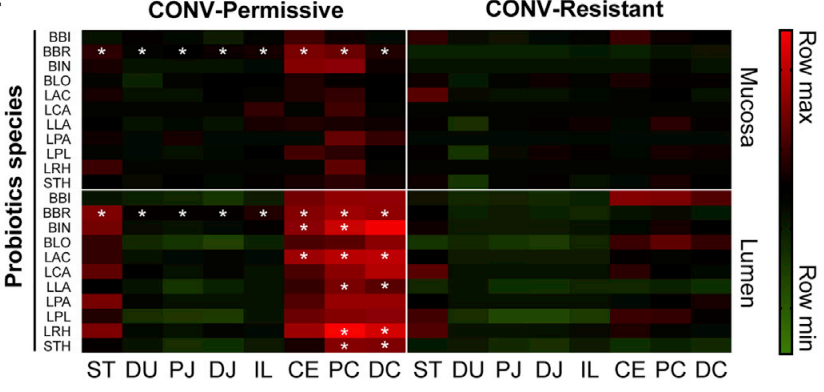
Researchers looked at the 11 strains of bacteria found in the probiotic and tested both the mucosa and lumen of 8 locations in the gut. The permissive group saw higher colonization numbers than the resistant group.
What they found is that some people’s guts were colonized by the probiotic, and some weren’t. Of the 10 people who received probiotics, 4 people did not experience significant colonization and 6 people experienced mild to significant colonization. The researchers labeled these groups as Resistant and Permissive. The resistant group did not experience colonization (their guts were resistant to the probiotic) and the permissive group did experience colonization (their guts permitted the probiotic to colonize).
What this shows us is that not everyone reacts to probiotics the same way.
The lesson is that probiotics are not a one size fits all solution.
Of the 10 people who took probiotics, 6 saw an increase in colonization, and 4 didn’t. It’s very important to remember here that these are 10 already healthy individuals. The most we can extrapolate is that if you’re already healthy, taking a probiotic has about a 60% chance of colonizing your gut.. However, 10 people is an extremely small sample size.
The researchers did find that there were certain immune characteristics distinct to the resistant and permissive groups. They hypothesized that in the future we may be able to run a simple test to determine how your body will respond to probiotics.
For now, clinicians must continue to look at the individual person, take into consideration any conditions and persistent symptoms, look at the person’s history, and make the most informed recommendation possible based on the individual person.
The second study asked “Does taking a probiotic after an antibiotic help repopulate the gut”
The second study published in the journal Cell is titled Post-Antibiotic Gut Mucosal Microbiome Reconstitution is Impaired by Probiotics and Improved by Autologus FMT (8).
You might be wondering, hasn’t someone already researched whether or not probiotics help after antibiotics? Yes, but they’ve been looking at stool samples as a representation of what’s happening in the gut. As we just saw from the first study, stool is not a mirror image of the gut microbiome. The researchers retested this hypothesis using the invasive methods to look at the what’s really happening inside the gut.
What previous studies found is that it can take a few months for the microbiome to stabilize after a round of antibiotics and the diversity might not fully recover (9). This is a concern because there are many unfriendly bacteria and viruses that will exploit the uninhabited digestive tract to colonize with pathogenic species. The idea has been to give probiotics after antibiotics so that pathogenic bacteria don’t have a chance to invade.
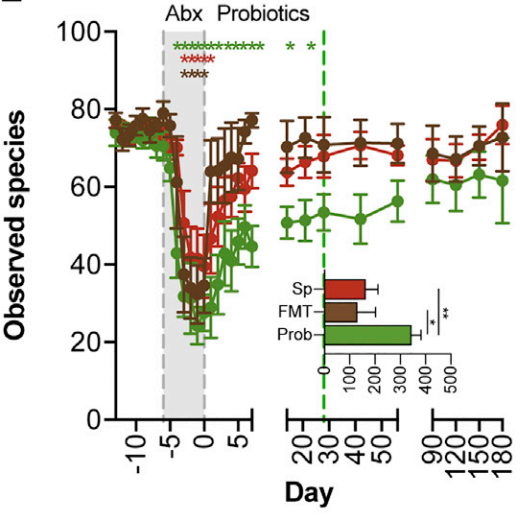
RED - spontaneous recovery group. No intervention given after antibiotic period. BROWN - Fecal Microbiota Transplant group. GREEN - Probiotic group. An 11 strain, 25 billion culture probiotic given 2x daily for 30 days post antibiotic treatment.
This graph is a good visual of what they found. The X axis shows a six month time period. The Y axis indicates species diversity. Species diversity of the microbiome is one of the characteristics of a healthy gut. Different species perform different jobs, so the higher the diversity the better.
Pink is the Spontaneous recovery group (n=7), so they took the antibiotic and received no intervention afterward.
Brown is the Fecal Microbiota Transplant group (n=6), so they saved a bit of poo before the study and used it to repopulate the person’s gut (10).
Green is the probiotic group (n=8). They received an 11 strain probiotic containing 25 billion active cultures twice daily for 30 days.
The graph shows that the FMT group was the first to recover higher diversity. An FMT intervention is like Noah’s Ark. They put a sampling of the original microbiome in a safe space, the antibiotic flood wipes everything out, and then they released the original population back into the wild. The diversity is recovered almost instantly and the bacteria are able to proliferate.
The spontaneous group catches up with the FMT group around day 40. The probiotic takes the longest to regain full species diversity. This makes sense too. If you take the Noah’s Ark idea, but instead of repopulating with a sample containing a wide diversity of organisms, you used a probiotic which contained about 11 species, you only populate with 11 species. The researchers theorized that the 11 species in the probiotic created too much competition for the native species to return in appropriate numbers.
Conclusion
This is really interesting data but it’s unclear how this will be clinically relevant. The study was performed on a very small sample size of already healthy individuals who took an antibiotic solely for the purpose of the study. We can only speculate how these results would translate for an individual taking a probiotic for a medical reason, or an individual with leaky gut, poor immune function, or a microbiome dysbiosis.
The researchers suggest looking further into some kind of personalized probiotics system, where instead of relying on a generic probiotic we have some way of understanding what your unique microbiome looks like and are able to repopulate accordingly.
They do note that the FMT had the best recovery times but it’s not an easy solution to implement. Oftentimes you don’t know when you’re going to need an antibiotic and so it’s difficult to plan ahead to have an FMT ready. On the other hand, this is definitely something to consider if it’s within your means and you do know ahead of time that you’ll be receiving antibiotics. If you have a surgery scheduled, if you’re pregnant and at risk for needing a c-section, this would be something to talk with your doctor about because you do have time to plan ahead in case you need it. We know that the transfer of bacteria from mother to baby is so important so that’s an area that would benefit from further study.
They also note that the only tested 1 type of probiotic, so something with higher diversity, different strains, could also cause different results. What would be really cool is to see this study replicated with an additional group of people eating traditional fermented foods after antibiotics.
Should you take a probiotic after antibiotics?
The study will definitely cause medical professionals to reconsider recommending probiotics after antibiotics. Certainly the person’s unique presentation needs to be taken into account, their symptoms, their history, how many round of antibiotics they’ve had. Studies have shown that your ability to recover after antibiotics does decrease every time you do another round.
It is also worth exploring how naturally fermented foods would fit into this situation. It is possible that a combination of spontaneous recovery, combined with a wide diversity of naturally fermented foods could speed up the recovery time. Additionally, limiting situations that increase your exposure to pathogenic species in the month after antibiotics seems prudent. Avoiding international travel, swimming in untested waters, and refraining from consuming raw or undercooked meat can reduce the chance of contracting a pathogen.
The most obvious lesson here, is one we already know. Antibiotics should be a last resort for a specific purpose. There are a lot of concerns about the over use of antibiotics and the creation of antibiotic resistant bacteria. Certainly that is a big take away here, is making sure we’re supporting the body with healthy food, addressing nutrient deficiencies, supporting healthy digestion as much as possible to really support the body so that antibiotics are not needed in the first place.